Crystal StructurE
When steel solidifies the atoms form into regular structures called crystals. These crystals form into groups called grains. Steel therefore has a grain structure and where the crystal structure changes we have a grain boundary.
Steel usually exists in one of two different crystal structures and it is these structures that give it its strength. The first is Body Centred Cubic (BCC) consisting of a cube with an atom at each corner and one in its centre. Materials with these structures are magnetic.
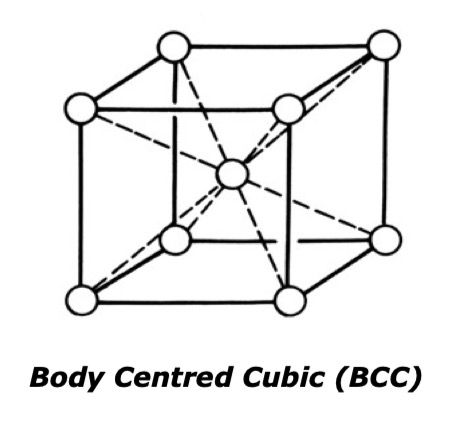
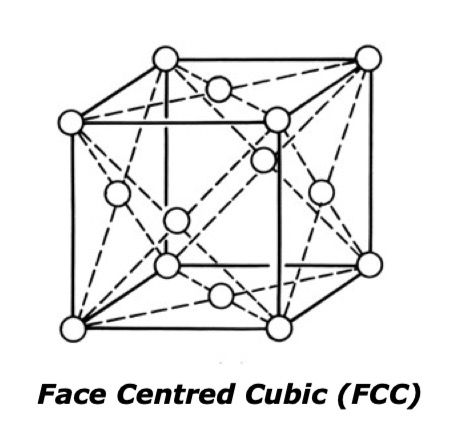
The second is the Face Centred Cubic (FCC). This also consists of a cube with atoms at each corner plus one in the centre of each face. The materials with this structure are non-magnetic. When steel is left to cool naturally, it changes from FCC back to BCC. This stage is known as solidification contraction and this is where the largest volume change can be seen.
Steel at room temperature may consist in a variety of forms depending on composition and heat treatment. Normally it will consist of a mix of ferrite and cementite, the mix giving hardness and ductility. Grains containing alternate layers of ferrite and cementite are called Pearlite.
The amount of the above constituents vary with carbon content.
Heat treatment can vary the hardness and other properties by changing the formation of the structure. An extreme case is that by rapid cooling, Martensite is formed which is very hard but brittle. (For further information see PRT-5)
Example crystal structures:
Carbon Steel – BCC
Aluminium - FCC Titanium –Hexagonal
Close Packed (HCP - refers to the most tightly packed or space-efficient
composition of crystal structures). Copper - FCC